Fauci, July 2020: On Ct values of PCR tests: At 4:40: “If you get a Cycle Threshold of 35 or more, the chances of it being replication competent are minuscule.” 5:00: “If someone does come in with 37, 38, even 36, you gotta say, you know, it’s just dead nucleotides, period.”
9/28/20, “Correlation Between 3790 Quantitative [PCR] Polymerase Chain Reaction–Positives Samples and Positive Cell Cultures, Including 1941 Severe Acute Respiratory Syndrome Coronavirus 2 Isolates,” Oxford Academic…[“Emergency” authorization for Covid PCR test in US expires on 12/31/21]
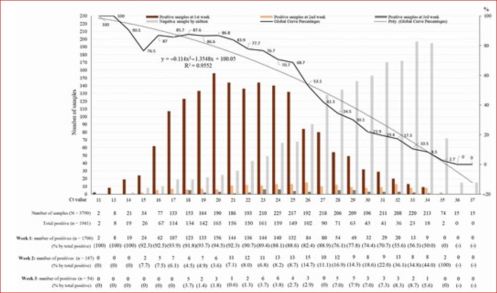
“High Ct values [in PCR tests] are mostly correlated with low viral loads."…
………………………..
Ct value greater than 28 needed for CDC vaccine analyses:
7/26/21, “COVID-19 Vaccine Breakthrough Case Investigation and Reporting,” cdc.gov/vaccines/covid-19
“This page provides information and resources to help public health departments and laboratories investigate and report COVID-19 vaccine breakthrough cases.”…
[scroll down, subhead] “COVID-19 vaccines are effective”
- Vaccine breakthrough cases occur in only a small percentage of vaccinated people. To date, no unexpected patterns have been identified in the case demographics or vaccine characteristics among people with reported vaccine breakthrough infections.
- COVID-19 vaccines are effective. CDC recommends that everyone 12 years of age and older get a COVID-19 vaccine as soon as they can.
- People who have been fully vaccinated can resume activities that they did prior to the pandemic.
- CDC would like to receive sequence data and respiratory specimens from COVID-19 vaccine breakthrough cases to assess the SARS-CoV-2 lineage, including variants. When a vaccine breakthrough case is identified, the health department will contact the laboratory to request that any residual respiratory specimen from the positive test be held for sequencing at CDC.
- The health department also will request the specimen ID numbers and the Ct value for positive RT-PCR results.
- If SARS-CoV-2 sequencing will not be performed locally and a
specimen is available, the state public health laboratory should request
the residual clinical respiratory specimen for subsequent shipping to
CDC.
- For cases with a known RT-PCR cycle threshold (Ct) value, submit only specimens with Ct value ≤28 to CDC for sequencing.
- If the Ct value is not known (e.g., positive by antigen test only or by a molecular test that does not provide a Ct value), the positive specimen may still be submitted to CDC for RT-PCR and potential sequencing.
- If your laboratory identifies a COVID-19 vaccine breakthrough case, please report it to your state health department so it can initiate the investigation with CDC.
- These instructions can also be found here: NS3 Submission Guidance Documents external icon.”
…………………………………..
Added: CDC wants no “weakly positive samples,” send samples of CT 28 or greater:
July 26, 2021, “Updated CDC Guidance for Specimen Submission for Surveillance of SARS-CoV-2,” David E. Wentworth, PhD, Lead – Strain Surveillance and Emerging Variants Team, COVID-19 Response Laboratory and Testing Task Force, Centers for Disease Control and Prevention, Enc: Appendix 1; Appendix 2; Appendix 3, FAQs…
“Appendix 1: National SARS-CoV-2 Strain Surveillance (NS3) Submissions to CDC for SARS-CoV-2 Positive Specimens,”
1. Beginning on Monday, August 2, 2021, CDC will accept SARS-CoV-2 positive specimens based on the revised guidance and processes below….
4. Acceptable specimen types for sequencing and potential virus characterization are the same as
for the CDC SARS-CoV-2 diagnostic assays that were authorized by FDA under an EUA: upper and lower respiratory specimens….
5. Considerations for selecting NS3 specimens:
a. The quality of the specimen directly affects sequencing and virus culture success. Ideally, specimens should have an RT-PCR Ct value of ≤28. If Ct values are not available, specimens that are positive/strong positive for SARS-CoV-2 may be sent (avoid weakly positive samples).”…
………………………………………….
Added: Linked on CDC website: “CDC prefers specimens with Ct values <28“
7/26/21, “National SARS-CoV-2 Strain Surveillance (NS3),” Association of Public Health Labs, aphl.org
“Thank you for your participation to help establish the US national SARS-CoV-2 genomic surveillance system….Below please find links to download specimen submission guidance and the specimen submission form.
Guidance Documents
CDC requests that state public health laboratories provide, on a weekly basis, confirmed, deidentified, diagnostic specimens to CDC to support the NS3 program. CDC prefers specimens with Ct values < 28 that have not already been sequenced. Specimens will ideally represent a variety of demographic and clinical characteristics and geographic locations. Selection of a diverse set of specimens will help ensure a representative set of sequences are generated for national monitoring.
Please see the documents below for additional details for submission guidance and shipping instructions:
- CDC Dear Colleague Letter on NS3 describing the project
- NS3 Frequently Asked Questions
- Guidance for submitting SARS-CoV-2 positive samples to CDC’s NS3 Program (Updated: July 26, 2021)
- Instructions for NS3 Reporting Dashboard
- Global File Accessioning Template (GFAT) password required (Updated: July 26, 2021)
- Supplemental Information for NS3 Submission Form password required (Updated: July 26, 2021)
- Questions?
For technical guidance on submission, interpretation or other technical sequencing related questions for SARS-CoV-2, please contact sarsseqshipping@cdc.gov.
For shipping account information or general questions on the national sequence-based surveillance strategy for SARS-CoV-2, please contact eoc@aphl.org.”
……………………………………
Added: 7/26/21, Screen shot of CDC website: “submit only specimens with Ct value ≤28 to CDC for sequencing.”


No comments:
Post a Comment